Brain Degenerative Diseases and Gut Flora
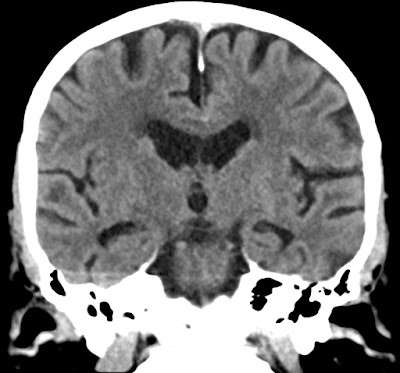
Coronal CT of 86 years old showing moderate atrophy i hippocampi - MTA 2.
Interesting articles indicate complex relation between our gut flora and neurodegenerative diseases. Especially in Alzheimer Dementia (AD).
*(citations from above articles in cursive font)Studies indicate that anaerobic bacteria producing toxins cause brain inflammation that disrupts fragile neural connections in the elderly. My private theory is that hygiene of the mouth plays important role. Environmental factors of the modern civilisation, food preservatives and destruction of natural gut flora plays important role. Also fact that people live longer allows more time for neurodegenerative diseases to develop.
One major class of microbiome-derived neurotoxin is the Gram-negative bacteria-derived lipoprotein glycoconjugate lipopolysaccharide (LPS) that has been reported by several independent research groups to reside within the brain cells and CNS tissues of aged patients affected with AD and in AD murine models (10–15). Many different variations of LPS are derived from different human microbiome-resident Gram-negative bacteria; for example, species such as the anaerobic bacterium Bacteroides fragilis are capable of secreting particularly pro-inflammatory and neurotoxic forms of LPS, such as BF-LPS, which penetrate physiological barriers, including brain cell plasma membranes (8–18). Importantly, Aβ peptides, one neuropathological hallmark for AD, have recently been shown to further support the translocation of LPS into neurons, probably via transient channel formation through the neuronal plasma membrane (6–12).
About important role of gut bacteria:
In recent years, human gut microbiota have become one of the most promising areas of microorganism research; meanwhile, the inter-relation between the gut microbiota and various human diseases is a primary focus. As is demonstrated by the accumulating evidence, the gastrointestinal tract and central nervous system interact through the gut–brain axis, which includes neuronal, immune-mediated and metabolite-mediated pathways. Additionally, recent progress from both preclinical and clinical studies indicated that gut microbiota play a pivotal role in gut–brain interactions, whereas the imbalance of the gut microbiota composition may be associated with the pathogenesis of neurological diseases (particularly neurodegenerative diseases), the underlying mechanism of which is insufficiently studied. This review aims to highlight the relationship between gut microbiota and neurodegenerative diseases, and to contribute to our understanding of the function of gut microbiota in neurodegeneration, as well as their relevant mechanisms.
Aging in humans is reportedly also related to significant shifts in the composition of gut microbiota, and the loss of microbial diversity was also evident in the aging gut. In addition, a striking alteration of microbiota composition was observed in the gastrointestinal tracts of elderly patients suffering from neurodegeneration [29]. Hence, researchers hypothesized that the alteration of human gut microbiota might well be one of the causes, or at least one of the contributing factors, of neurodegenerative diseases.
Currently, the prevalence of neurodegenerative disease is rapidly rising. Although genetic susceptibility is a major risk factor of neurodegenerative diseases, environmental factors throughout one’s lifetime also exert a great influence on the onset, development and eventual severity of such diseases.
According to a recent comparative study, human AD patients had a decreased microbial diversity, compared to both healthy controls and patients with merely mild cognitive impairments (MCI).
Moreover, intestinal bacteria can also secrete a large number of lipopolysaccharide (LPS) and amyloid proteins. On one hand, these substances can directly enter the brain through the intestinal and blood–brain barriers. On the other hand, they can also induce a series of inflammatory reactions and increase the permeability of these barriers [39]. Additionally, the microbial amyloid protein produced by gut microbiota can also interact with the Toll-like receptor TLR2 to induce the activation of pro-inflammatory mediators such as interleukin (IL-17A, IL22), subsequently inducing an immune response and stimulating the production of the amyloid protein in the brain neurons [40].
Important role plays intestinal epithelium being a major barrier for the penetration of our organism by harmful bacteria and their toxins.
Antibiotics in food destroy natural bacteria flora that has protective function.
Indigenous microbiota in the intestinal tract is the first line of defense against invading exogenous pathogens [54]. Specifically, the beneficial microorganism species contribute to the prevention of intestinal infection through mechanisms such as altering the pH value of the intestinal microenvironment, secreting anti-bacterial substances, and directly competing for adhesion sites or nutrition on the epithelium surface [55,56,57]. In clinical scenarios, antibiotics-related diarrhea occurs mostly when the treatment with antibiotics starts to substantially disrupt the natural balance between the intestinal microbiota subpopulations and leads to the proliferation of harmful bacterial types (e.g., Clostridium difficile).
Important role is played by probiotics:
Additionally, Lactobacillus GG (a probiotic species) significantly shortened the disease course of infectious diarrhea in infants and children [59]. Both results suggested that certain types of gut microbiota might have played a substantial role in the systematic reaction against gut-mediated infection.
Importance of the main bacteria in our mouth - Lactobacillus salivarius - present in our saliva:
Specifically, Lactobacillus salivarius and Bifidobacterium breve are considered important bacteria species that contribute to the stabilization of the immune system.
Important role play vitamins that are necessary for our good bacteria to thieve.
There are even hypothesis that bacteria can be the cause for multiple sclerosis (MS):
Bacteria is occasionally capable of passing through the blood–brain barrier, or the blood–cerebrospinal fluid barrier, to enter the central nervous system, the mechanisms of which include trans-cellular infiltration, paracellular entering, or via the infected leukocytes [130]. Branton et al. [131] detected the existence of bacteria in the brain tissue of multiple sclerosis (MS) patients and discovered that Proteobacteria were the dominant flora in the cerebral white matter of female MS patients, a phenomenon that is reportedly associated with the expression of inflammation-related genes in patients’ brains.
Importance of good mouth hygiene - chronic gingival inflammation stimulates growth of harmful bacteria:
Studies also found that Porphyromonas gingivalis, a primary pathogen of chronic periodontitis, existed in the brain tissue of patients with Alzheimer’s disease.
Now we know why our dogs eat poop 💩🐶:
Fecal Microbiota Transplantation (FMT) - FMT refers to the transplantation of feces containing gut microbiota from healthy donors to recipients with dysbacteriosis, by means of an enema or nasogastric, nasointestinal, or endoscopic approaches, aiming to restore the normal diversity and functionality of the gut microbiome [157,158]. This method is currently considered an effective treatment for the recurrent infection of Clostridium difficile.